

This means that for this membrane potential, the electrical force due to the potential difference and the chemical force due to the concentration difference (or osmotic difference) are equal and act in opposite directions. The electrochemical potential of an ion is zero at the level of the equilibrium/reversal potential (also known as the Nernst potential), when the value of the membrane potential is in electroosmotic equilibrium. The excess protons are then flushed through the ATP-synthase during oxidative phosphorylation and the formation of ATP. The mitochondrial membrane is impermeable to protons in the direction of the matrix, therefore more protons accumulate here than in the matrix itself. This energy, stored in the form of chemical potential, is stored by the process of oxidative phosphorylation into ATP for later use. The difference in chemical concentrations can be compared to the potential energy available for work inside the cell. The tendency of an electrically charged particle to pass through a membrane depends on the difference in electrochemical potentials on each side of the membrane.

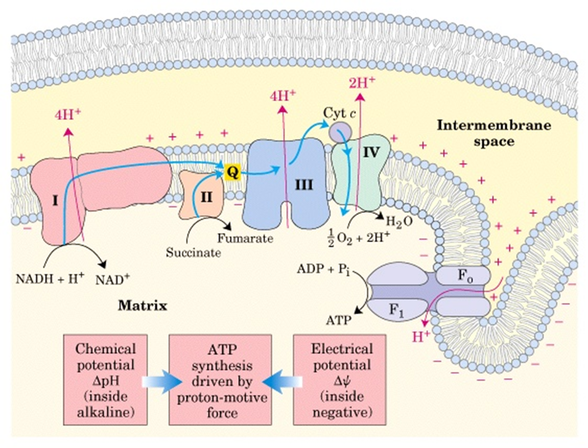
From the difference in chemical concentrations on both sides of the membrane.From the electrical potential that is caused by the change in charge across the lipid membrane.Thus, the electrochemical potential itself consists of two parts.Δϕ is the electric potential across the membrane (in V) Ψ=− Δϕ + (2,3 RT/F) log (C cation,ex / C cation,in) Where: Electrochemical potential is defined as:.In biological processes, it determines the direction in which this ion or proton will move, either by diffusion or active transport. Electrochemical potential is therefore a gradient of ions or protons (electrochemical gradient) that can move across the membrane.


 0 kommentar(er)
0 kommentar(er)
